
TECHNOLOGY
‘Electrolyte’, the best driver for lithium-ion
ALL > Technology
I will drive safely and fast for lithium-ions! ‘Electrolyte’
A lithium-ion battery uses liquid electrolytes. It allows lithium ions(Li+) move between anode and cathode, stabilizes cathode and anode surfaces, extends battery lifespan, and improves cell performances. It plays a pivotal role as one of the major four components of a battery.
In a nutshell, liquid electrolytes serve as ‘a medium that enables movement of lithium ions’ between cathode and anode. We can say it is the best driver that drives fast and safely for lithium ions.
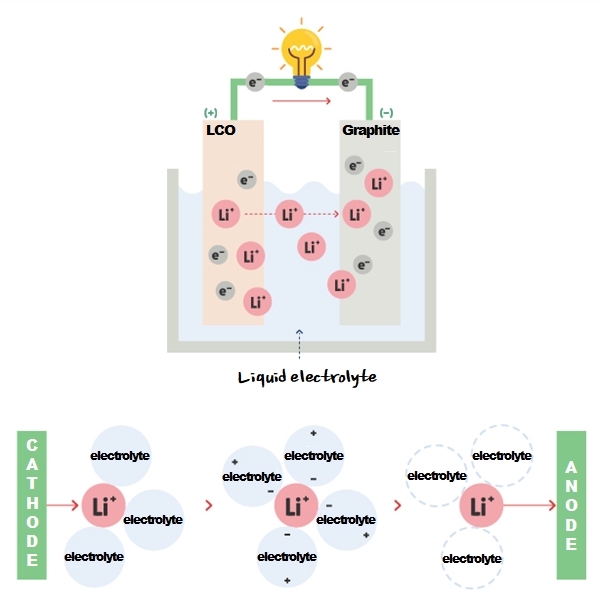
Liquid electrolytes are consisted of lithium salt, organic solvent, and additives. Let’s take a look at each one of them.
Lithium salt, passage of lithium ions
Lithium salt serves as a passage for lithium ions. So, lithium ions should be easily dissolved or dissociated in a solvent, and those dissociated ions will move smoothly.
* Dissociation : Chemical compounds are split into ions
The most commonly used lithium salt is LiPF6(composed of lithium·phosphate·fluorine), and it offers better ion movement, dissolution, and higher chemical stability.
Organic solvent, liquid that dissolves lithium salt
Organic solvent dissolves lithium salt and helps lithium ions travel easily. There are some requirements for organic solvents. Organic solvents should have high solubility for lithium salt to separate ionic compounds, and low viscosity for smooth movement of lithium ions.
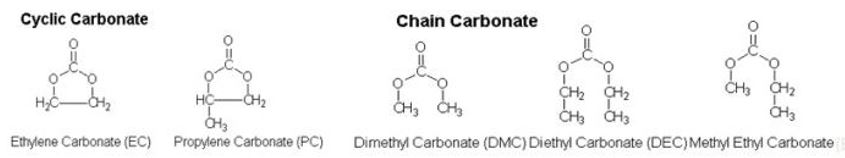
[ Various types of solvents ]
It varies by solvents, but in order to have high ionic conductivity, we should have the combination of cyclic carbonate with high solubility for lithium salt, and chain carbonate with low viscosity.
* Ionic conductivity : Efficiency of ion’s movements
And a solvent should have low chemical reactivity. Lithium often responds to water, so the solvent of liquid electrolytes should not respond to water.
Additive, a substance that decides liquid electrolytes
Additive is a substance added in small quantity, forming a protective film on cathode and anode surfaces. It helps smooth movement of lithium ions between cathode and anode, and prevents battery degradation.
Additives are divided into two: Cathode and anode additives. Cathode additives stabilize a cathode structure and protect the surface to prevent battery aging, thus reducing overheating and overcharging.
Anode additives are dissolved earlier than a solvent, creating a strong film in the anode to increase lifespan, prevent overheating and maintain battery capacity.
Both cathode and anode additives should be well dissolved in liquid electrolytes and chemically stable, and we use different additives according to client’s required specifications or different purposes.
Additives take only a small part of the liquid electrolyte, but it plays a crucial role in the entire system by increasing lifespan, improving high-temperature issues, and reducing resistance.
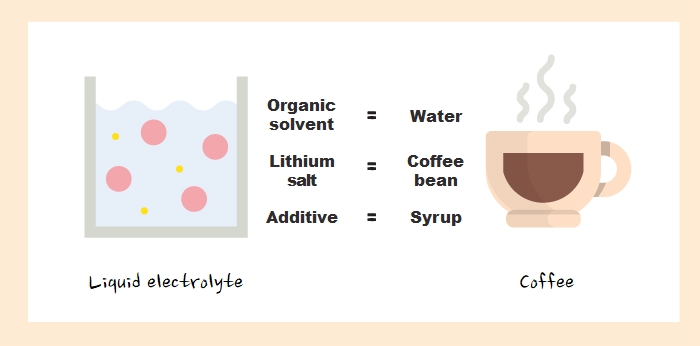
We’ve learned about the electrolyte. It seems difficult in theory, but if you compare the liquid electrolyte to coffee, you’ll easily understand it. You can think this way. Lithium salt is coffee bean, organic solvent is water, and additive is syrup.
Introduce me a good electrolyte!
Liquid electrolyte is ‘a medium that moves lithium ions fast and safely’, so it should be able to move ions smoothly. And it should be chemically∙electrically stable not to undermine battery performance. Because in some cases, liquid electrolytes have side reaction while the battery is operated. The freezing point should be low and the boiling point should be high so that the battery can operate anytime.
One of the hot topics today in the battery industry is ‘a solid-state battery’, the next-generation battery.
[Structures of lithium-ion battery(left) and solid-state battery(right)]
Solid-state battery uses solid electrolytes, not liquid electrolytes, so it is safer than other batteries without fire risks.
It is not commercialized yet, but we will be able to use it in our daily lives as Samsung SDI is making a great effort to commercialize it!










