
TECHNOLOGY
What is the secret behind a safe and long-lasting battery? The answer is anode materials!
ALL > Technology
Lithium-ion in cathode? I (anode) can take all of you!
Lithium-ion generates electricity by flowing in and out of both cathode and anode. And a lithium-ion battery is charged when absorbing the electricity, and discharged when releasing it.
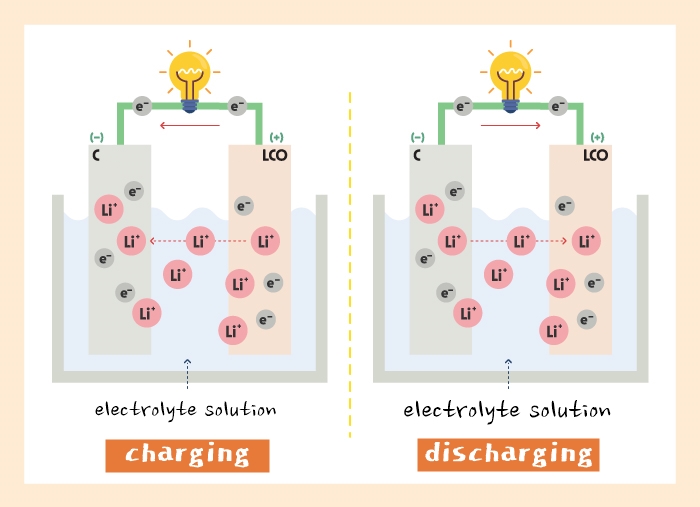
Let’s say that cathode is a home, anode is a restaurant, and lithium-ion is a customer. Charging occurs when a customer (lithium-ion) leaves home (cathode) and goes to a restaurant (anode) to eat, and discharging occurs when a customer who finished eating leaves a restaurant and goes back to home.
But what if the restaurant doesn’t have enough seats? The restaurant made more seats at outdoor patio, but what if the seats are too close to the road with many vehicles? In that case, the customer cannot eat at the restaurant, the restaurant cannot sell food, and the outdoor patio could be at risk.
Accordingly, if there is no safe place for lithium-ion, we cannot secure capacity or power of cathode. However, if we design anode well, we can have a battery with higher capacity and longer lifespan.
What’s important? Battery lifespan? Capacity? Don’t forget anode
Anode is the most important part to extend the battery lifespan. Graphite is used as an anode material. Graphite has a layered structure where carbon atoms in each layer are bonded. And if charging a battery, lithium-ion flows from cathode to anode, and goes into each gap between graphite layers. Here, the graphite with lithium-ion expands a little.
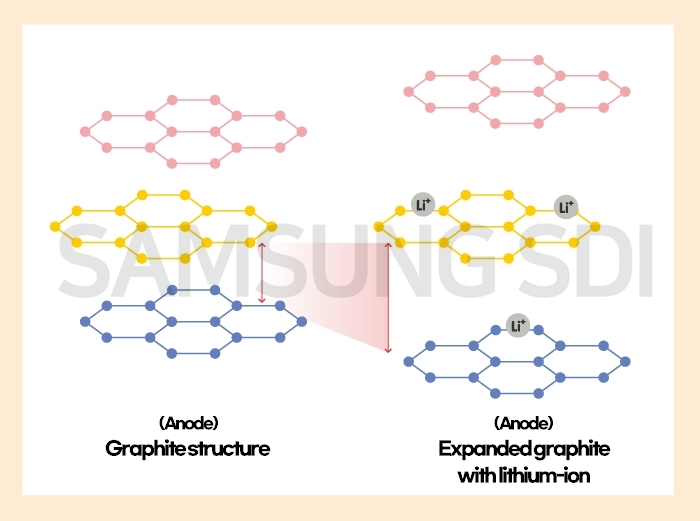
A lithium-ion battery can be used continuously by going through a charging and discharging cycle, but the battery lifespan decreases over time. It is because of break-down in anode structure caused by degradation of anode which absorbs lithium-ion. Every product, no matter how strong it is, becomes old and wears down over time. And the same goes for batteries. Changes in anode size are not safety issues, because they are natural phenomenon.
Change in anode volume also affects capacity to some extent. We consider structural changes inside a battery when designing a battery. If we use materials that expand easily, we cannot predict size changes and make enough spaces, which leads low capacity. On the other hand, if we use materials that do not expand easily, we can make enough spaces, thus increasing battery capacity.
Next-generation anode material, Silicon(Si)
Graphite is the most commonly used anode material, followed by silicon(Si). Silicon offers 10 times higher energy density than graphite, and high charging/discharging speed.
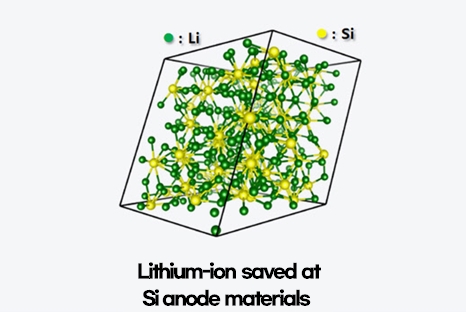
However, silicon still needs to be improved. The expansion. If lithium-ion flows into anode, graphite-based anode expands by 10%, but silicon-based anode expands by 400%. It is true that silicon has 10 times higher energy density than graphite, but we should first stabilize the unstable structure of silicon-based anode. Accordingly, the battery industry is carrying out R&D to stabilize the structure of silicon-based anode.
Samsung SDI has developed a ‘super-gap’ battery by using our silicon anode material
As silicon is getting much attention as next-generation anode material, it is an urgent task to reduce expansion of silicon. So, Samsung SDI has developed and commercialized ‘SCN(Si-Carbon-Nanocomposite)’, one of our patented technology. SCN is a ‘composite’ material that combines graphite and ‘nano-sized’ silicon that is smaller than one-thousandth the width of a human hair. We came up with the idea to combine advantages of silicon and graphite.
We combined structurally stable graphite and nano-sized silicon with high energy density, so we could develop a safe and high energy density material.
With more cordless electronic devices out in the market, the demand for longer battery runtime is increasing. If we combine high-nickel cathode and silicon-based anode material, we can increase battery runtime, lifespan, and have quick charge. Samsung SDI will pursue a ‘super-gap’ strategy to develop battery technologies.










